
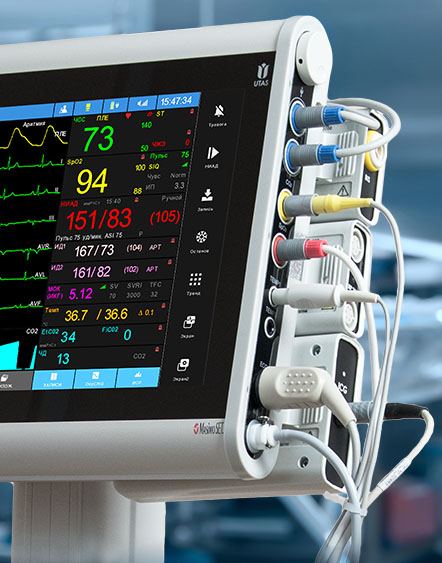
Medical devices are classified based on the risks associated with the use of the device. Devices are classified as Class I, Class II, or Class III — with Class I being the lowest risk and Class III the highest risk.
Class I – These are devices that present minimal potential for harm to the user. Examples include enema kits and elastic bandages. 35% of medical device types are Class I and 93% of these are exempt from pre-market review.
Class II –These are devices that generally present a moderate risk of harm to the user. Examples of Class II devices include powered wheelchairs and some pregnancy test kits. 53% of device types are Class II, most of which require FDA review through premarket notification (510(k)).
Class III – These are devices that sustain or support life, are implanted, or present potential high risk of illness or injury. Examples of Class III devices include implantable pacemakers and breast implants. 9% of device types are Class III and require FDA review through premarket approval (PMA) or humanitarian device exemption (HDE).
Unclassified/Not classified – These are device types that FDA has not yet classified. 3% of device types are unclassified/not classified.
Some devices may be considered “exempt”. If a device is considered “exempt”, the manufacturer would not be required to submit a premarket notification submission [510 (k) submission] and obtain FDA clearance before marketing the device in the U.S.


Morbi et dolor est. Donec at dolor vehicula, molestie erat non, rutrum tellus. Vestibulum in eros…
Lorem ac mauris et dui tempor blandit. Donec neque ligula, accumsan ac rutrum quis, lacinia sed urna. Lorem ipsum dolor set.