

To get a PSA (prostate-specific antigen) test, while checking for prostate cancer, a patient would need to get blood drawn in a lab, and wait for the results, and then make a follow up appointment to discuss the results.
Claros uses microfluidics technology, which allows for the manipulation of fluids on a chip at microscopic scales, to develop inexpensive, easy-to-use diagnostic devices that can be deployed at the point of care using only a finger stick drop of blood. We helped them with how the technology could be used without needing a trained phlebotomist on site. Claros has successfully validated the technology for urology and infectious disease market applications, and this validation will serve as a bridge to other test panels for infectious disease, cardiology, women’s health, companion diagnostics, and almost any field of blood diagnostics.
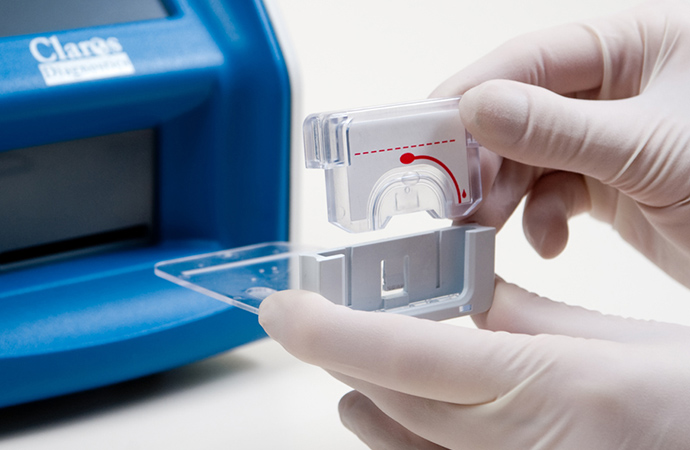
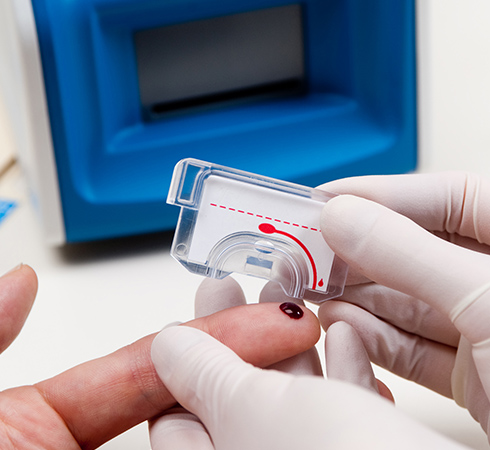
The key to a project that changes the paradigm of blood based tests as much as Claros did is making the collection of the blood sample as easy as possible. Traditional blood sampling methods require a visit to a lab for filling a Vacutainer® of blood to send to a lab, but as the Claros device can use a much smaller sample it gave us the opportunity to use a simple finger prick and capillary tube to get enough blood for analysis.
We developed an easy to hold capillary tube that had indicated to the user when it had sufficient blood collected, and enclosed that in a housing that would snap into the disposable test cassette, ready for analysis. The complete system was designed so a user could get a suitable sample with minimal training.
The desktop analyzer contained all the hardware to pull the sample of blood into the microfluidic channels for analysis. We designed the analyzer to have a large screen that would show step-by-step instructions to minimize errors.
Throughout the development process we did user trials with nursing assistants and doctors, at our in house observation and usability suite to verify that the device met FDA and IEC 60601 requirements